Home Tags Posts tagged with "food and drug administration"
food and drug administration
If you’ve ever had a prescription filled in the United States, you’re aware that American drug prices can be ridiculously high. But some drugs are pricier than others — and health insurance providers are fighting back.
The recently released prescription drug Solvadi (sofosbuvir) is a case in point. Solvadi is the only cure for hepatitis C, a liver infection that can lead to cirrhosis, liver cancer, liver failure and ultimately death. You’d think this drug would be a godsend for the many patients struggling with hepatitis C — until you got a look at the price.
 Solvadi costs a staggering $1,000 a pill, or $84,000 for a full, 12-week course of treatment. Other specialty drugs, like those used to treat rheumatoid arthritis, cancer and multiple sclerosis can cost patients an average of $10,000 a month. Even drugs for more common conditions can be prohibitively expensive for most people, which is why many Americans turn to online pharmacies like Medicines Mexico to cut their prescription drug costs.
Solvadi costs a staggering $1,000 a pill, or $84,000 for a full, 12-week course of treatment. Other specialty drugs, like those used to treat rheumatoid arthritis, cancer and multiple sclerosis can cost patients an average of $10,000 a month. Even drugs for more common conditions can be prohibitively expensive for most people, which is why many Americans turn to online pharmacies like Medicines Mexico to cut their prescription drug costs.
Health insurance providers say drug prices that high simply can’t be borne. Many insurers are now refusing to cover the costs of expensive drugs at all, at least in cases where the drug’s perceived benefit doesn’t justify its price, or there are other drugs available that do the same thing for less money. Patients will be forced to use similar drugs with lower price tags.
Prescription Drug Access Is a Problem for Many Americans
Prescription drug sales topped out at $326 billion in the 12 months prior to September 2013, and Americans’ prescription drug spending is expected to grow by three to five percent by the end of 2014. The high price of prescription drugs is to blame, and physicians around the nation are aware that, for most people, affording prescription drugs is difficult, especially when it comes to obtaining some of the most expensive treatments.
Jerry Avorn, a Harvard Medical School professor and chief of the Division of Pharmacoepidemiology and Pharmacoeconomics at Brigham and Women’s Hospital, told CNBC, “Access really is a problem for a lot of patients in relation to these very costly medicines. We sometimes think, ‘Well, people have insurance, and under Obamacare there’s a lot more coverage,’ but what that doesn’t take into account is that very often there is a very big co-payment that the patient has to come up with that is often many, many hundreds or thousands of dollars.”
In 2012, Memorial Sloan-Kettering Cancer Center chose to stop prescribing the costly cancer medication Zaltrap (ziv-aflibercept) on the grounds that the drug didn’t provide any additional benefit over older drugs, despite its substantially higher cost. In response, the drug’s French manufacturer, Sanofi, cut the price of Zaltrap in half.
Insurers Forcing Drug Price Negotiations
While the U.S. Centers for Medicare and Medicaid services are prohibited by law from attempting to negotiate lower drug prices, health insurance providers are not. With rising health care prices affecting everyone, insurance providers are now giving pharmaceutical manufacturers a choice. Either drug manufacturers can slash the prices of their most expensive drugs, or insurers will remove those drugs from their formularies. A drug not listed in an insurance provider’s formulary won’t be covered under any plans — patients who want it will have to pay full list price out of their pockets.
Of course, health insurance providers aren’t going to dump drugs just because they’re expensive. If there’s no equivalent drug available yet — such as in the case of Solvadi — insurers will likely still cover it regardless of cost. But patients are already being asked to switch to less expensive equivalents of the medications they need — CVS Caremark dumped around 30 expensive drugs from its formulary in 2012 and 70 more this year; next year’s formulary will exclude 200 drugs. Express Scripts dumped 48 drugs and medical products this year. A new Catamaran formulary is 54 drugs lighter this year. Some of the drugs dumped include popular prescriptions like Advair (fluticasone/salmeterol) and Victoza (liraglutide).
So far, American drug manufacturers haven’t responded by lowering the prices they charge insurance companies. Nor will the strategy affect list prices, the prices paid by patients who must buy their drugs out of pocket, without help from an insurance provider. List prices will remain the same. Instead, insurers hope that by dumping costly drugs or forcing manufacturers to bargain, they can keep premium costs low.
Drug prices are astronomical, especially for some specialty drugs used to treat cancer, hepatitis C, rheumatoid arthritis and multiple sclerosis. Insurance providers, in a bid to keep premiums manageable, are dumping expensive drugs by the dozens. Unless drug manufacturers decide to start bargaining with insurers, patients will find their prescription drug options dwindling by the year.
The FDA has banned India’s largest drugmaker Ranbaxy from producing and distributing drugs for the US market from its Toansa facility in Punjab.
The agency said there had been “significant” manufacturing violations at the facility.
The FDA alleged that staff had retested materials after those items had failed initial tests “in order to produce acceptable findings”.
Other Ranbaxy units have also come under scrutiny by the US in the past.
The FDA has previously banned products from the company’s facilities in Paonta Sahib, Dewas and Mohali.

The FDA has banned Ranbaxy from producing and distributing drugs for the US market from its Toansa facility in Punjab
“We are taking swift action to prevent substandard-quality products from reaching US consumers,” Carol Bennett, acting director of the FDA’s Office of Compliance, said in a statement.
Ranbaxy said in a statement that it had “voluntarily and proactively suspended shipments” from the facility to the US after it received the inspection findings earlier this month.
“This development is clearly unacceptable and an appropriate management action will be taken upon completion of the internal investigation,” said Arun Sawhney, chief executive of Ranbaxy.
Ranbaxy’s shares fell as much as 20% on the FDA ruling.
[youtube lNTyUkrHFkY 650]
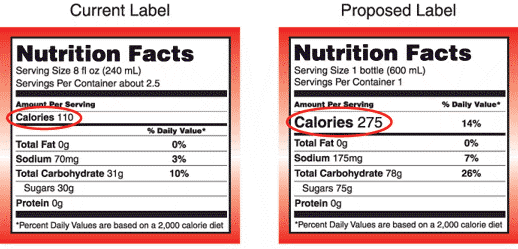
The FDA says knowledge about nutrition has evolved over the last 20 years, and food labels need to reflect that.
Those nutrition labels on the back of food packages may soon become easier to read.
As the agency considers revisions, nutritionists and other health experts have their own wish list of desired changes.
The number of calories should be more prominent, the agency says, and the amount of added sugar and percentage of whole wheat in the food should be included. They also want more clarity on how serving sizes are defined.
“There’s a feeling that nutrition labels haven’t been as effective as they should be,” says Michael Jacobson of the Center for Science in the Public Interest.
“When you look at the label, there are roughly two dozen numbers of substances that people aren’t intuitively familiar with.”
For example, Michael Jacobson says, most of the nutrients are listed in grams, the metric system’s basic unit of mass. He says people don’t really understand what a gram is.
Michael Taylor, the FDA’s deputy commissioner for foods, says 20 years ago “there was a big focus on fat, and fat undifferentiated”. Since then, health providers have focused more on calories and warned people away from saturated and trans fats more than all fats. Trans fats were separated out on the label in 2006.
The nutrition facts label “is now 20 years old, the food environment has changed and our dietary guidance has changed,” says Michael Taylor, who was at the agency in the early 1990s when the FDA first introduced the label at the behest of Congress.
The FDA says knowledge about nutrition has evolved over the last 20 years, and food labels need to reflect that
“It’s important to keep this updated so what is iconic doesn’t become a relic.”
The FDA has sent guidelines for the new labels to the White House, but Michael Taylor would not estimate when they might be released. The FDA has been working on the issue for a decade, he said.
There’s evidence that more people are reading the labels in recent years.
According to an Agriculture Department study released this month, a greater percentage of adults reported using the nutrition facts panel and other claims on food packages “always or most of the time” in 2009 and 2010 compared with two years earlier.
The USDA study said 42% of working adults used the panel always or most of the time in 2009 and 2010, while older adults used it 57% of the time during that period.
One expected change in the label is to make the calorie listing more prominent, and Regina Hildwine of the Grocery Manufacturers Association said that could be useful to consumers. Her group represents the nation’s largest food companies.
Regina Hildwine said FDA also has suggested that it may be appropriate to remove the “calories from fat” declaration on the label.
It’s not yet clear what other changes the FDA could decide on. Nutrition advocates are hoping the agency adds a line for sugars and syrups that are not naturally occurring in foods and drinks and are added when they are processed or prepared. Right now, some sugars are listed separately among the ingredients and some are not.
It may be difficult for the FDA to figure out how to calculate added sugars, however. Food manufacturers are adding naturally occurring sugars to their products so they can label them as natural – but the nutrition content is no different.
Other suggestions from health advocates:
— Add the percentage of whole wheat to the label. Many manufacturers will label products “whole wheat” when there is really only a small percentage of it in the food.
— Clearer measurements. Michael Jacobson of CSPI and others have suggested that the FDA use teaspoons instead of grams on the label, since consumers can envision a teaspoon.
— Serving sizes that make sense. There’s no easy answer, but health experts say that single-size servings that are clearly meant to be eaten in one sitting will often list two or three servings on the label, making the calorie and other nutrient information deceptive. FDA said last year that it may add another column to the labels, listing nutrition information per serving and per container. The agency may also adjust recommended serving sizes for some foods.
— Package-front labeling. Beyond the panel on the back, nutrition experts have pushed for labels on the package front for certain nutrients so consumers can see them more easily. The FDA said several years ago it would issue guidelines for front of pack labeling, but later said it would hold off to see if the industry could create its own labels.
The Food and Drug Administration (FDA) has warned that antibacterial chemicals in soaps and body washes may pose health risks.
The FDA called for a safety review of such products.
The agency proposed a rule requiring manufacturers to prove such soaps are safe and more effective against infection than plain soap and water.
Recent studies indicate an ingredient in such products could scramble hormone levels and boost drug-proof bacteria.
Manufacturers have until the end of 2014 to submit the results of clinical trials on their products, the FDA said. The new regulations would be finalized in 2016.
“New data suggest that the risks associated with long-term, daily use of antibacterial soaps may outweigh the benefits,” Colleen Rogers, an FDA microbiologist, wrote in a statement on Monday.

The FDA has warned that antibacterial chemicals in soaps and body washes may pose health risks
Certain ingredients in such products – such as triclosan in liquid soaps and triclocarban in bar soaps – may contribute to bacterial resistance to antibiotics, the agency added.
Such products may also have “unanticipated hormonal effects that are of concern”, according to the statement.
Recent studies of such chemicals on animals have shown they may alter hormones, the FDA said, but such results have not yet been proven in humans.
“Because so many consumers use them, FDA believes that there should be clearly demonstrated benefits to balance any potential risks,” the statement added.
If the FDA’s proposed rule is finalized, companies would be required to provide data to support their product’s health claims.
If they cannot, the products would be reformulated or relabeled in order to remain on the market.
In March, a federal appeals court approved a lawsuit by the non-profit Natural Resources Defense Council, aimed at forcing the FDA to review the health impacts of triclosan.
[youtube 587DlseljNg 650]
The FDA has imposed a ban on 23andMe, a company offering personal genetic screening to the general public.
23andMe has been ordered to “immediately discontinue” selling its saliva-collection tests after failing to provide information to back its marketing claims.
The tests aim to show how personal genetic codes may affect future health.
The company said it would address concerns.
The start-up has been operating since 2006 and was co-founded by Anne Wojcicki, the wife of Google co-founder Sergey Brin.
For $99, users receive a kit allowing them to take sample of saliva. This is sent to the company and in return users receive a readout of their genetic code.
The website promises reports on 254 health conditions and traits as well as offering to help people trace their genealogy.

23andMe has been ordered to “immediately discontinue” selling its saliva-collection tests after failing to provide information to back its marketing claims
Under FDA rules, the company must provide proof about how accurate its detection methods are as well as supplying the error rates from its personal genome service (PGS).
In a public letter, the FDA said that 23andMe had not supplied this information, despite increasing its marketing campaign and the scope of its tests.
“FDA is concerned about the public health consequences of inaccurate results from the PGS device – the main purpose of compliance with FDA’s regulatory requirements is to ensure that the tests work,” said Alberto Gutierrez, director of the FDA’s centre for devices and radiological health, in a letter to the company.
“Patients relying on such tests may begin to self-manage their treatment through dose changes or even abandon certain therapies depending on the outcome of the assessment,” he added.
Despite hundreds of emails and 14 face-to-face meetings with 23andme, little evidence had been provided, the agency added.
The company said: “We recognize that we have not met the FDA’s expectations regarding timeline and communication regarding our submission.
“Our relationship with the FDA is extremely important to us and we are committed to fully engaging with them to address their concerns.”
The Center for Genetics and Society said it welcomed the FDA’s stance.
“Our society regulates medical products to protect public health. Without strong public oversight, we’re back to the era of snake oil,” said executive director Marcy Darnovsky.
“The public agency charged with protecting public health has finally lost patience with a private company that seems to think it doesn’t have to play by the rules,” she added.
There are an increasing number of companies offering low-cost home genetic testing – but some medical experts have raised questions about the accuracy of the tests, and asked what benefit they offer to consumers.
[youtube _agiFqtCE4U 650]
Cola’s color comes in part from 4-methylimidazole (4-MEI or 4-Mel), a chemical that forms in the production of caramel food coloring.
Coca-Cola, Pepsi and other manufacturers insist it is safe at the low doses found in drinks.

Cola’s color comes in part from 4-methylimidazole, a chemical that forms in the production of caramel food coloring
But studies have shown that long-term exposure to the chemical causes lung cancer in rats, and health officials in California ruled that products with more than 29mcg must carry a health warning.
When research by the Center for Science in the Public Interest, a campaign group, found cans contained nearly 140 mcg, all Cola companies across the U.S. were forced to cut levels.
Food campaigners say daily consumption of 4-MI at 30 mcg would cause cancer in one in 100,000 people over their lifetimes.
But the U.S. Food and Drug Administration says that someone would need to drink more than 1,000 cans of cola every day to reach the levels that caused cancer in lab rats.
[youtube hzdex3Ejb9Y]
Harmful levels of lead, far higher than regulations suggest are safe, have been revealed following an analysis of the commercially available rice imported into the US.
Some samples exceeded the “provisional total tolerable intake” (PTTI) set by the Food and Drug Administration (FDA) by a factor of 120.
The report at the American Chemical Society Meeting adds to the already well-known issue of arsenic in rice.
Lead is known to be harmful to many organs and the central nervous system.
It is a particular risk for young children, who suffer significant developmental problems if exposed to elevated lead levels.
Because rice is grown in heavily irrigated conditions, it is more susceptible than other staple crops to environmental pollutants in irrigation water.

Harmful levels of lead have been revealed following an analysis of commercially available rice imported into the US
Recent studies have highlighted the presence of arsenic in rice – prompting consumption advice from the UK’s Food Standards Agency and more recently from the FDA.
However, other heavy metals represent a risk as well.
Dr. Tsanangurayi Tongesayi of Monmouth University in New Jersey, and his team have tested a number of imported brands of rice bought from local shops.
The US imports about 7% of its rice, and the team sampled packaged rice from Bhutan, Italy, China, Taiwan, India, Israel, the Czech Republic and Thailand – which accounts for 65% of US imports.
The team measured the lead levels in each country-category and calculated the lead intake on the basis of daily consumption. The results will be published in the Journal of Environmental Science and Health (Part B).
“When we compared them, we realised that the daily exposure levels are much higher than those PTTIs,” said Dr. Tsanangurayi Tongesayi.
“According to the FDA, they have to be more than 10 times the PTTI levels [to cause a health concern], and our values were two to 12 times higher than those 10 times,” he said.
“So we can only conclude that they can potentially cause harmful effects.”
That factor of 120 is for Asian children, who are most susceptible by virtue of age and comparatively high rice intake on average.
For non-Asian adults the excesses above the PTTI ranged from 20 to 40.
Rice from China and Taiwan had the highest lead levels, but Dr. Tsanangurayi Tongesayi stressed that all of the samples significantly exceeded the PTTIs.
He has also worked on quantifying arsenic contamination – and is in effect working his way through the heavy metals one by one to determine their prevalence.
The problem, he said, is the range of agricultural practices around the world.
“If you look through the scientific literature, especially on India and China, they irrigate their crops with raw sewage effluent and untreated industrial effluent,” he explained.
“Research has been done in those countries, and concerns have been raised because of those practices, but it’s still ongoing.”
Dr. Tsanangurayi Tongesayi also said that the increasing practice of sending electronic waste to developing countries – and the pollution it leads to – exacerbates the problem.
“With a globalised food market, we eat food from every corner of the world, but pollution conditions are… different from region to region, agricultural practices are different from region to region, but we ignore that.
“Maybe we need international regulations that will govern production and distribution of food.”
So far, such international oversight exists informally in the form of the Codex Alimentarius, a collection of food-safety standards first set out by the UN.
FDA spokesman Noah Bartolucci said the “FDA plans to review the new research on lead levels in imported rice released today”.
“As part of an ongoing and proactive effort to monitor and address contaminants in food traded internationally, FDA chairs an international working group to review current international standards for lead in selected commodities, including rice, and to revise, if necessary, maximum lead levels under the… Codex Alimentarius,” he said.
AeroShot producer, Breathable Foods Inc., has received a warning letter for false or misleading statements in the labeling of its product, the U.S. Food and Drug Administration announced on March 6.
There are concerns about the safety of the “caffeine inhaler” and about children and adolescents using it combined with alcohol, said the FDA.
AeroShot gives “breathable energy, anytime, anyplace,” and it is intended to be ingested by swallowing, it is written on the product website, but the FDA said these statements were in contradiction.
“The company’s labeling is false or misleading because these two claims contradict each other. A product cannot be intended for both inhalation and ingestion because the functioning of the epiglottis in the throat keeps the processes of inhaling and swallowing separate,” said the FDA.
Also, labeling a product as “breathable energy” may lead to an improper usage.
Consumers may tend to inhale it, but inhaling caffeine into the lungs is not safe as long this process has not been well studied. The particles in AeroShot are too big to enter the lungs, says the company on its website, but it shows no specific research in support of this claim.

On March 6, 2012, the FDA issued a warning letter to the makers of AeroShot “caffeine inhaler.”
In the same time the FDA is worried about the use of the AeroShot by the children.
AeroShot is “not recommended for those under 18 years of age,” says the website and it is “not intended for people under 12,” says the label.
“But the website also appears to target these age groups by suggesting it be used when studying,” said the Agency.
The product is not intended for persons under 18, said Tom Hadfield, Breathable Foods CEO.
“We plan to work closely with the FDA to meet their requests for information and labeling changes to ensure compliance with dietary supplement requirements. AeroShot delivers a mix of B vitamins and caffeine to the mouth for ingestion and is not ‘inhaled’ into the lungs,” he told the Huffington Post.
The FDA has another concern regarding the utilization of AeroShot while drinking alcohol.
The product website has links to news articles and videos about using AeroShot in combination with alcohol. Those news items express health concerns about this kind of usage, but their presence publicizes and may encourage the use of AeroShot with alcohol, said the FDA.
Taking caffeine while drinking may make consumers to feel “less drunk,” but it does not lower blood alcohol levels, and it does not remove the side effects of drinking too much alcohol. Mixing caffeine with alcohol can be dangerous, especially for very young people, old people, or persons with health problems.
The product label does not include contact information for consumers to report adverse events, as required under federal law.
Consumers who believe they have suffered illness or injury from using AeroShot should also report those events to their regional FDA Consumer Complaint Coordinators. The agency also encourages healthcare providers to report to FDA any adverse events in their patients that are associated with AeroShot. As of March 6, 2012, FDA has not received any adverse event reports associated with the product.
In 2010 there was another issue over caffeinated energy drink Four Loko. The media coverage at that time led to increased sales and the drink became more popular. However, the caffeine was eliminated from Four Loko and now further restriction may be added.
AeroShot is sold out on the company’s website, but remains available on store shelves in Boston and New York, according to Breathable Foods.
Senator Chuck Schumer, of New York, promised to oppose AeroShot, over two months ago. The FDA has to take measures concerning the product, because the children and adolescents may abuse it, he said.
Massachusetts-based company, Breathable Foods Inc. has 15 business days to respond to the agency with a plan to bring the AeroShot into compliance with FDA regulations.
 Solvadi costs a staggering $1,000 a pill, or $84,000 for a full, 12-week course of treatment. Other specialty drugs, like those used to treat rheumatoid arthritis, cancer and multiple sclerosis can cost patients an average of $10,000 a month. Even drugs for more common conditions can be prohibitively expensive for most people, which is why many Americans turn to online pharmacies like Medicines Mexico to cut their prescription drug costs.
Solvadi costs a staggering $1,000 a pill, or $84,000 for a full, 12-week course of treatment. Other specialty drugs, like those used to treat rheumatoid arthritis, cancer and multiple sclerosis can cost patients an average of $10,000 a month. Even drugs for more common conditions can be prohibitively expensive for most people, which is why many Americans turn to online pharmacies like Medicines Mexico to cut their prescription drug costs.






