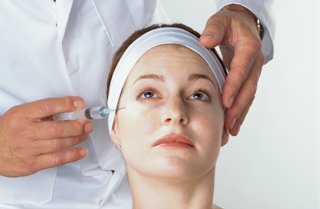
Botox treatment for wrinkles around the corners of the eyes – also known as “crow’s feet” – was officially sanctioned by the Food and Drug Administration on Wednesday.
Crow’s feet are thought to be caused by repeated muscle contractions over a lifetime through facial expressions like frowning, smiling and squinting.
“This additional indication will provide people with a new FDA approved treatment option for those seeking a smoother appearance by temporarily minimizing the appearance of crow’s feet at the sides of the eyes,” said Susan Walker, director of the Division of Dermatology and Dental Products in the FDA’s Center for Drug Evaluation and Research, in the FDA statement.
Botox has been used for decades by opthalmologists to treat eyelid twitching, or blepharospasm, according to Dr. Jennifer Walden.
The Texas-based aesthetic plastic surgeon, told CBSNews.comthat this is one of the ways doctors discovered it could smooth the skin.
While this new FDA approval for Botox only came Wednesday, Dr. Jennifer Walden said: “Doctors have been using it off-label for years to smooth the crow’s feet.”
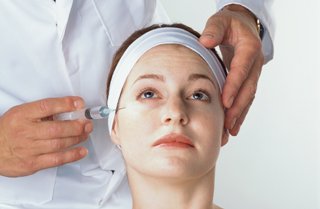
The new FDA approval came following a study of 833 adults conducted by the drug’s manufacturer, Allergan Inc. of Irvine, California, where participants were randomly assigned to receive a Botox or a placebo injection.
People treated with Botox had greater reductions in crow’s feet.
Botox (onabotulinumtoxinA) is a protein and neurotoxin and is the most acutely toxic substance known.
However, when injected directly into a muscle in approved doses, Botox prevents muscles from tightening by temporarily paralyzing them, which in turn makes wrinkles disappear – for up to six months.
Botox was first approved as a cosmetic by the FDA in 2002 for temporary improvement of frown lines – the wrinkles between the eyebrows, also known as glabellar lines.
The most common side effect seen was eyelid edema, which causes the eyelids to become swollen due to fluid buildup.
Botox labels contain the strongest warning the FDA offers saying the effects of the toxin may spread from the area of injection to other areas of the body.
However, the FDA said there has never been a confirmed serious case of this happening when Botox is used at recommended doses.
According to Dr. Marco Harmaty, a plastic and reconstructive surgeon at The Mount Sinai Medical Center in New York City, Botox has been frequently used for crow’s feet and other unapproved areas of the face, like the side of the nose.
The FDA’s new approval “does give you an added benefit and safety of saying that I’m not doing anything illegal or potentially harmful”, he said to CBSNews.com.
Botox for cosmetic purposes still likely won’t be covered by insurers, given it is used for cosmetic purposes.
Insurers often cover people who have approved medical conditions treated by Botox, including chronic migraines, severe underarm sweating and others.