A new combination of drugs had positive effects in hepatitis C genotype 1 infection in a high percent of patients who had not responded to previous treatment, according to a study published on New England Journal of Medicine on January 19.
This report came from Phase II of a pilot study funded by Bristol-Myers Squibb and led by Anna S. Lok, M.D., professor of Internal Medicine, Division of Gastroenterology at the University of Michigan Medical School.
“The two recently approved hepatitis C drugs – telaprevir or boceprevir — combined with PEG-interferon alfa and ribavirin have limited success in patients who have not responded to previous treatment with PEG-interferon alfa and ribavirin. Because of this high unmet medical need, there is a necessity for new combination regimens that can increase response rates in that population. The high rate of sustained virologic response in patients who received the four drug regimen is very exciting. Although only four of 11 patients given the two direct-acting antiviral agents only achieved sustained virologic response, this is the first study to show that sustained virologic response can be achieved without the use of interferon or ribavirin. These data are very encouraging because PEG-interferon alfa and ribavirin are associated with many side effects and many patients with hepatitis C choose not to receive treatment for fear that they cannot tolerate those drugs,” said Dr. Lok.
The clinical trial enrolled patients with chronic hepatitis C, who had not responded to previous treatment with PEG-interferon alfa and ribavirin.
Twenty-one patients with chronic hepatitis C virus (HCV) genotype 1 infection, who had not responded to previous treatment with PEG-interferon alfa and ribavirin after more than 12 weeks of treatment were enrolled.
They randomly received a combination of two investigational direct-acting antiviral agents (daclatasvir and asunaprevir) alone, or were given this combination along with PEG-interferon alfa-2a and ribavirin.
In the group that received the combination alone were assigned 11 patients, 4 of them (2 of 9 with HCV genotype 1a and 2 of 2 with genotype 1b) had a sustained virologic response at 12 weeks after treatment and at 24 weeks after treatment, 6 (with HCV genotype 1a) had viral breakthrough while receiving therapy, and resistance mutations to both antiviral agents were found in all cases; 1 patient had a viral response at the end of treatment but had a relapse after the treatment period.
In the group that received daclatasvir, asunaprevir, PEG-interferon alfa-2a and ribavirin were assigned 10 patients. They all had a sustained virologic response at 12 weeks after treatment, and 9 had a sustained virologic response at 24 weeks.
A sustained virologic response or SVR means there is no detectable hepatitis C virus in a patient’s blood after treatment is stopped. Achieving sustained virologic response is important, because research has shown that late relapse is rare.
“Overall, these results suggest that further research into combinations of direct-acting antiviral agents, with or without PEG-interferon and ribavirin, should be encouraged. Caution must be exercised in selecting the right combination of direct-acting antiviral agents in studies of interferon-free regimens because in this study, all 7 patients who received only two direct-acting antiviral agents that did not achieve sustained virologic response had emergence of drug resistance variants to both drugs,” said Dr. Lok.
Diarrhea was the most common adverse event in both groups. Six patients had temporary elevations of alanine aminotransferase (ALAT, a hepatic enzyme that rise when the liver is damaged) levels to more than 3 times the upper limit of the normal range.
Daclatasvir is a NS5A replication complex inhibitor daclatasvir and was administered 60 mg once daily. Asunaprevir is a NS3 protease inhibitor and was given 600 mg twice daily.
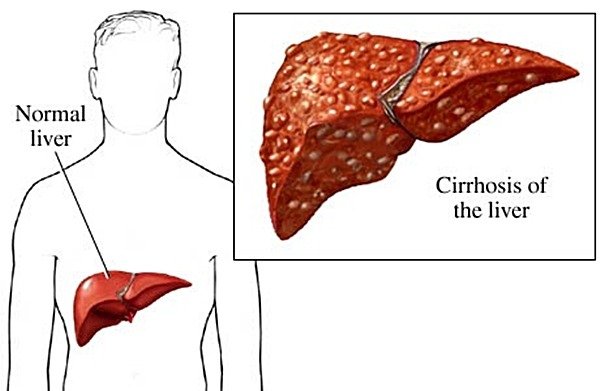
Hepatitis C genotype 1 is the most common type in the United States and the most difficult to treat.
This disease is caused by a virus (hepatitis C virus, or HCV) that infects the liver and it is transmitted through direct contact with infected blood and blood products, transfusions of infected blood, using unsterilized needles or other contaminated instruments. Hepatitis C is extremely rare transmitted through sexual intercourse.
Worldwide around 170 million people are infected with hepatitis C, especially with genotype 1. The chronic infection occurs in almost 80% of them. Twenty percent of persons with chronic hepatitis C are at risk of developing cirrhosis and, of those, up to 25 percent may progress to liver cancer. There is no vaccine to prevent hepatitis C, but this is a potentially curable disease.