Biopharmaceutical company Oxigene has presented data that showed Zybrestat (fosbretabulin) helped improve the one-year survival rate of patients with larger anaplastic thyroid cancer tumors.
The presentation was made on September 14 at the 35th Meeting of the European Thyroid Association, in Krakow, Poland. The presenter was Dr. Rossella Elisei, endocrinologist, University of Pisa, Italy.

A comparison was made between the results of the company’s aggregate data from five independent prospective Phase 1 and Phase 2 trials using Zybrestat to treat patients with anaplastic thyroid cancer (ATC), including the FACT trial, and data from the 50-year experience in treating ATC patients at the Mayo Clinic from 1949 to 1999, published by Dr. Bryan McIver. FACT trial is a large single, randomized, controlled, multi-center clinical trial, and the Mayo studies comprise a large retrospective review of the treatment outcomes in ATC.
There were three key findings from the retrospective analysis.
13 patients or 9.7% of all 134 ATC patients treated at the Mayo Clinic survived one year or more.
19 patients or 23% of all 84 ATC patients treated with Zybrestat either alone or in combination with chemotherapy survived more than one year. The longest survival is 13+ years in a patient with a complete response.
The 9% rate of patients in the control arm of the FACT study surviving one year is almost identical to the observed 9% in the larger Mayo cohort.
Direct comparisons between the FACT trial and the Mayo Group have to be interpreted with the caveat that there were changes in standard-of-care treatment over the past 50 years, as well as differences in staging, sample size, and time-to-death.
“The 24% of patients treated with Zybrestat in the FACT study surviving one year or longer remains striking in its significance. Patients treated with Zybrestat essentially had a one-in-four chance of being alive after one year compared to only a one-in-10 chance in the control group, which corresponds to the survival outcome in the Mayo Clinic treated group.” Said Dr. Peter J. Langecker, Oxigene’s CEO.
Oxigene earlier this month saw shares slump after it said funding for a planned Phase 3 study of the drug candidate wasn’t feasible. Langecker said the company believes focusing its clinical resources on its most promising early-stage clinical development opportunities and reducing operating costs will allow it to conserve available resources.
Previously referred to as combretastatin A4 phosphate/CA4P, Zybrestat (fosbretabulin) has been granted orphan drug status by the FDA and the European Medicines Agency for the treatment of anaplastic thyroid cancer.
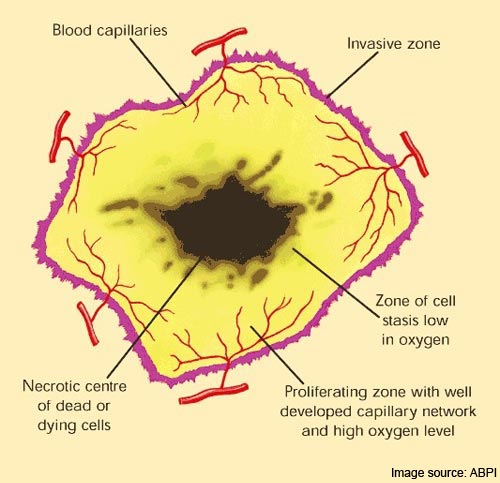
Zybrestat is being evaluated in a Phase 2 study of patients with non-squamous non-small cell lung cancer and other clinical trials, including a recently completed Phase 2 study of Zybrestat plus chemotherapy in patients with ATC. Oxigene believes that Zybrestat is poised to become an important product in a novel class of small-molecule drug candidates called vascular disrupting agents (VDA). Through interaction with vascular endothelial cell cytoskeletal proteins, Zybrestat selectively targets and collapses tumor vasculature, thereby depriving the tumor of oxygen and causing death of tumor cells. In clinical trials in solid tumors, Zybrestat has demonstrated potent and selective activity against tumor vasculature, as well as clinical activity against anaplastic thyroid cancer, ovarian cancer and various other solid tumors.

The concept of interrupting tumors’ blood supply was first described in the early 1970s and led to the development of a new class of anti-cancer drugs called angiogenesis inhibitors.
Zybrestat is the first VDA to be tested in combination with a tumor-angiogenesis-inhibiting drug Avastin (bevacizumab) in humans.
The thyroid is an endocrine gland and its hormones help control heart rate, blood pressure, body temperature, and weight. Four main types of thyroid cancer are papillary, follicular, medullary, and anaplastic thyroid cancer.

Anaplastic thyroid cancer is a very aggressive primary thyroid malignancy. ATC represents less than 2% of all thyroid cancers, but causes up to 40% of deaths from thyroid cancer. Median life expectancy is about three months for newly diagnosed patients. The overall 5-year survival rate of ATC has been given as 7% or 14%.
National Cancer Institute estimates 48,020 new cases and 1,740 deaths from thyroid cancer in the U. S. in 2011.
Fosbretabulin (Zybrestat), bortezomib (Velcade) and TNF-Related Apoptosis Induced Ligand (TRAIL), are being trialed in clinical labs and human clinical studies.