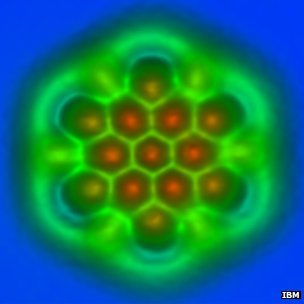
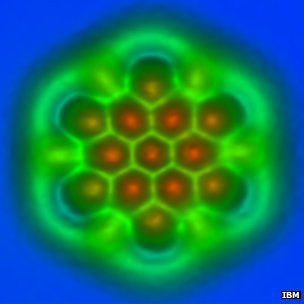
An IBM team in Zurich has published single-molecule images so detailed that the type of atomic bonds between their atoms can be discerned.
The same pioneering team took the first-ever single-molecule image in 2009 and more recently published images of a molecule shaped like the Olympic rings.
The new work opens up the prospect of studying imperfections in the “wonder material” graphene or plotting where electrons go during chemical reactions.
The images are published in Science.
The team, which included French and Spanish collaborators, used a variant of a technique called atomic force microscopy (AFM).
AFM uses a tiny metal tip passed over a surface, whose even tinier deflections are measured as the tip is scanned to and fro over a sample.
The IBM team’s innovation to create the first single molecule picture, of a molecule called pentacene, was to use the tip to pick up a single, small molecule made up of a carbon and an oxygen atom.
This carbon monoxide molecule effectively acts as a record needle, probing with unprecedented accuracy the very surfaces of atoms.
It is difficult to overstate what precision measurements these are.
The experiments must be isolated from any kind of vibration coming from within the laboratory or even its surroundings.
They are carried out at a scale so small that room temperature induces wigglings of the AFM’s constituent molecules that would blur the images, so the apparatus is kept at a cool -268C.
While some improvements have been made since that first image of pentacene, lead author of the Science study, Leo Gross, said the new work was mostly down to a choice of subject.
The new study examined fullerenes – such as the famous football-shaped “buckyball” – and polyaromatic hydrocarbons, which have linked rings of carbon atoms at their cores.
The images show just how long the atomic bonds are, and the bright and dark spots correspond to higher and lower densities of electrons.
Together, this information reveals just what kind of bonds they are – how many electrons pairs of atoms share – and what is going on chemically within the molecules.
“In the case of pentacene, we saw the bonds but we couldn’t really differentiate them or see different properties of different bonds,” Dr. Leo Gross said.
“Now we can really prove that… we can see different physical properties of different bonds, and that’s really exciting.”
The team will use the method to examine graphene, one-atom-thick sheets of pure carbon that hold much promise in electronics.
But defects in graphene – where the perfect sheets of carbon are buckled or include other atoms – are currently poorly understood.
The team will also explore the use of different molecules for their “record needle”, with the hope of yielding even more insight into the molecular world.